Introduction
One of the most aggressive types of acute myeloid leukemia (AML) is caused by aberrant activation of EVI1 as a result of the hijacking of enhancers repositioned by structural rearrangements. One of these enhancers, the MYC-blood enhancer cluster ( MYC-BENC) (Bahr et al. 2018) activates EVI1 in AML patients with t(3;8). MYC-BENC consists of several enhancer modules, of which only one is essential for activating EVI1: deletion of this element abrogates MYC-BENC to EVI1 promoter interaction as well as transcription of EVI1 (Ottema et al. 2021).
Approach
Given the central role of acetyltransferases p300 and CBP in enhancer activation, in particular super-enhancers such as MYC-BENC, we wondered whether we could specifically inhibit MYC-BENC driven EVI1 transcription using p300/CBP inhibitors. We studied this in a previously reported t(3;8) K562 model (Ottema et al. 2021), in which we introduced GFP 3' of EVI1 as a readout for MYC-BENC-driven EVI1 expression.
Results
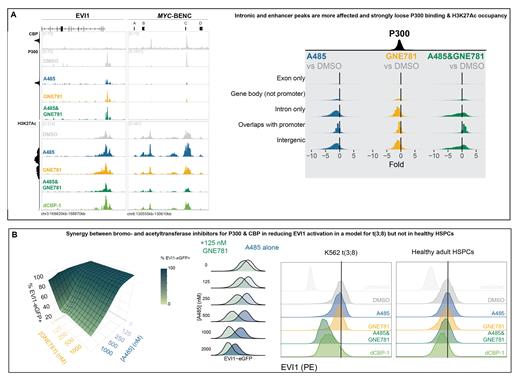
ChIP-seq in t(3;8) cells showed that p300 binds to the promoter of EVI1 and to MYC-BENC, whereas CBP only binds to MYC-BENC (Fig. A). Following rapid p300/CBP degradation (3 hours) with the heterobifunctional p300/CBP degrader dCBP-1 (Vannam et al. 2021), we found a severe reduction of nascent EVI1 (and MYC) transcripts (SLAM-seq), as well as of EVI1 protein levels, i.e. by EVI1-eGFP, intracellular anti-EVI1-PE staining (Fig. B) and western blot.. Importantly, p300/CBP degradation was accompanied by loss of H3K27ac at MYC-BENC, but not at the promoter of EVI1 (Fig. A). Thus, the activation of EVI1 in t(3;8) AML appears strongly dependent on the presence of p300/CBP at MYC-BENC super-enhancer. Importantly, in healthy HSPCs, we observed that EVI1 expression was not strongly downregulated upon p300/CBP degradation (Fig. B). These data suggest that MYC-BENC driven EVI1 transcription in AML can be targeted, while leaving EVI1 transcription in normal HSPCs unaffected.
We next studied whether loss of enhancer activity was dependent on functional domains within p300/CBP using specific inhibitors of either the acetyltransferase domain (A485) or bromodomain (GNE781). Treatment of t(3;8) EVI1-GFP cells with single inhibitors had a mild effect on EVI1 expression, but simultaneous A485/GNE781 treatment showed strong synergy in reducing EVI1 levels (Fig. B). Dual inhibition also led to a profound loss of H3K27ac signal at the MYC-BENC locus, but not at the EVI1 promoter (Fig. A). Thus, both domains in CBP/p300 are required to activate EVI1 expression via the hijacked MYC-BENC enhancer. As with dCBP-1, we found that EVI1 expression levels in healthy HSPCs were not affected by A485 and GNE781 treatment (Fig. B). We hypothesized that A485/GNE781 treatment would only cause loss of p300/CBP activity, but p300 ChIP-sequencing showed that A485/GNE781 treatment caused strong loss of p300 binding at the enhancer while the total protein levels of p300 and CBP were not affected. p300 binding at the EVI1 promoter was not decreased by A485/GNE781 treatment. In line with this, we observed that genome-wide p300 binding at enhancers was reduced at 15939 out of 41160 (39%) putative enhancers upon A485/GNE781 exposure, whereas dual inhibitor treatment only affected p300 binding at 429 out of 12452 promoters (3.5%) (Fig. A), suggesting a high selectivity of p300 inhibitors for long distant regulatory elements.
Conclusion
In summary, EVI1 is sensitive to pharmacological p300/CBP inhibition when activated by the hijacked MYC-BENC enhancer, but not in normal HSPCs. Globally, the sensitivity of genomic regions to bromo- or acetyltransferase inhibition is highly locus-dependent and is mainly restricted to enhancers. Our findings demonstrate that aberrantly controlled expression of oncogenes by hijacked enhancers in leukemia can be pharmacologically targeted, pointing to a potential therapeutic avenue for patients who are refractory to current treatment regimens.
Disclosures
No relevant conflicts of interest to declare.